A proven treatment
with established safety1
A proven treatment
with established safety1
Safety profile
Safety and tolerability data reflect exposure to ARCALYST in more than 2000 patients.1
These included patients with cryopyrin-associated periodic syndromes (CAPS) and recurrent pericarditis, patients with other diseases, and healthy volunteers.
- Included approximately 151 patients exposed for at least 6 months1
- Included 111 patients exposed for at least 1 year1
In the Phase 3 trial, RHAPSODY, injection-site reactions* and upper respiratory tract infections were the most common adverse events associated with use of ARCALYST.2
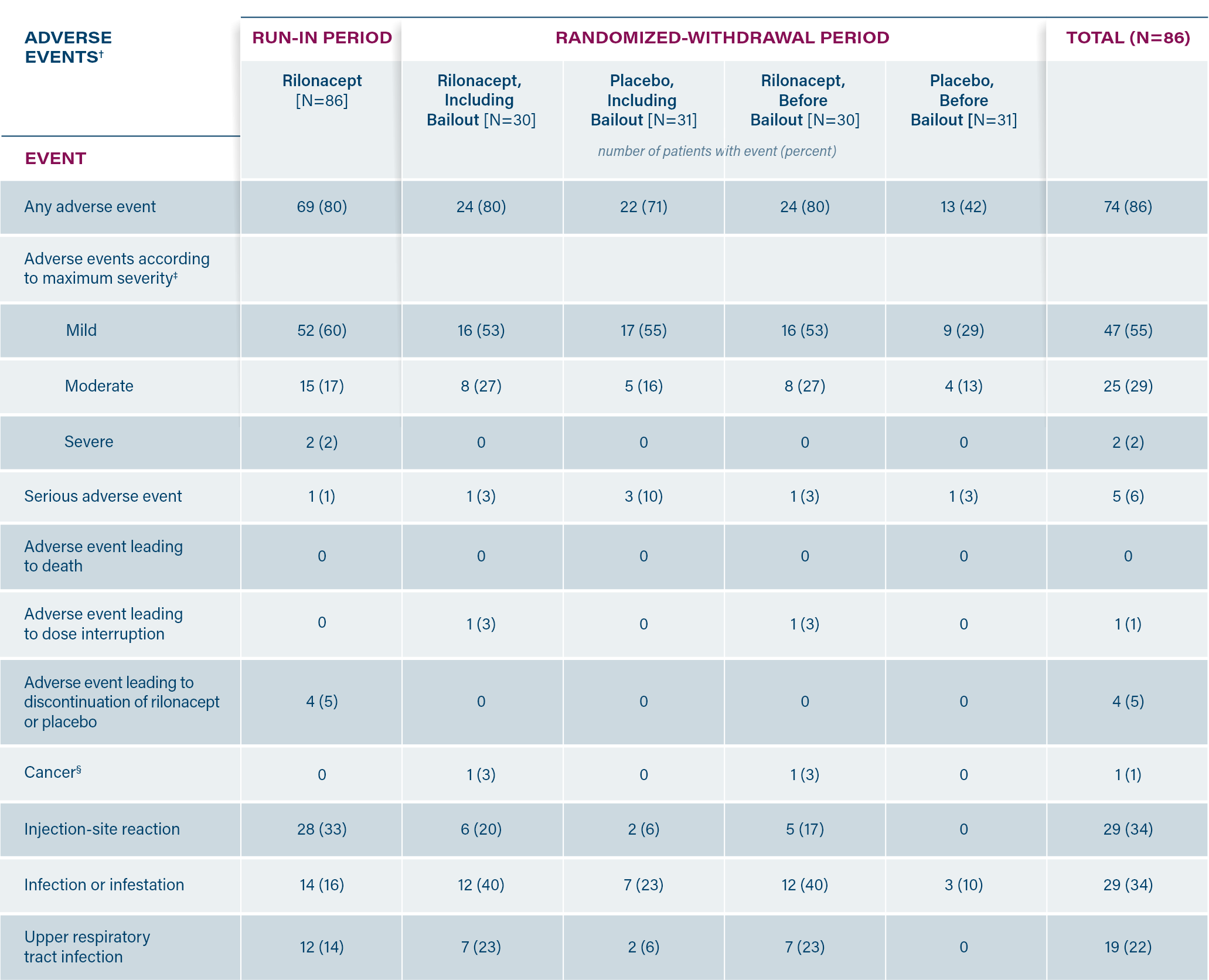

*Including erythema, swelling, pruritus, mass, bruising, inflammation, pain, edema, dermatitis, discomfort, urticaria, vesicles, warmth and hemorrhage.1
†Patients with multiple events were counted once in each appropriate category.
‡Counted once, according to the maximum severity of the adverse event.
§Cancer was an event of special interest.
DOSING AND ADMINISTRATION
ARCALYST is a once-weekly subcutaneous therapy.1
Loading dose
ARCALYST should be initiated with a loading dose that is 2x the dose of the following weekly maintenance doses.
The loading dose of ARCALYST should be performed under the supervision of a healthcare professional.
Maintenance dose
The weekly maintenance dose can be self-administered by the patient.
Adults (18 years and older)
Loading dose:
320 mg
given as two 160-mg (2 mL) injections
Weekly maintenance dose:
160 mg
given as a once-weekly 2-mL injection
Adolescents (12 to 17 years)
Loading dose:
4.4 mg/kg
given as 1 or 2 injections, up to a maximum of 320 mg (up to 2 mL)
Weekly maintenance dose:
2.2 mg/kg
given as a once-weekly injection, up to a maximum of 160 mg (up to 2 mL)
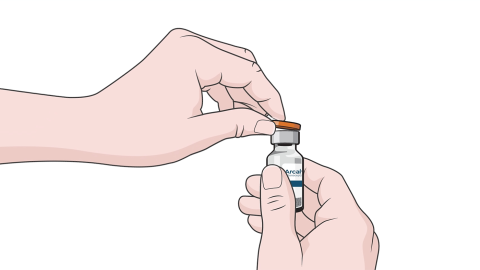
STEP-BY-STEP ADMINISTRATION
ARCALYST reconstitution and injection
follow a step-by-step process.1
Patients should receive training from a healthcare professional before self-administering
ARCALYST for the first time.

ARCALYST is supplied in sterile, single-use, 20-mL glass vials.1
- Each vial contains 220 mg rilonacept, a sterile, white to off-white, lyophilized powder
- Reconstitution with 2.3 mL of preservative-free Sterile Water for Injection is required prior to subcutaneous administration of the drug
- The reconstituted ARCALYST is a viscous, clear, colorless to pale yellow, free from particulates, 80-mg/mL preservative-free solution

Take an in-depth look at
how to use ARCALYST.
DURATION OF TREATMENT
The duration of a patient's ARCALYST treatment will be determined by the prescribing physician.

Real-world evidence indicates that the mean duration of recurrent pericarditis could be up to 3 to 4 years from the first episode—longer in patients with a greater burden of recurrences3
- In RHAPSODY, the mean duration of disease at study entry was 2.4 years1
In RHAPSODY, the median duration of rilonacept treatment was 9 months2
- At the completion of the RW period, 74 of 75 eligible patients chose to enroll in the ongoing long-term extension (LTE) treatment period.2
At the 1-year anniversary of the start of the LTE treatment period, the median duration of continuous rilonacept therapy was approximately 20 months4